Understanding the molar mass of CaCO3 is essential for anyone studying chemistry, environmental science, or materials engineering. Calcium carbonate (CaCO3) is a compound that plays a significant role in various natural and industrial processes. From forming the structure of marine organisms to being a key ingredient in cement production, its applications are vast. This article will explore the concept of molar mass, how it applies to CaCO3, and why it matters in scientific and practical contexts.
Calcium carbonate is a chemical compound with the formula CaCO3. It is one of the most abundant minerals on Earth, found in rocks, shells, and even in our daily lives as chalk or limestone. Knowing the molar mass of CaCO3 is crucial for performing stoichiometric calculations, determining reaction yields, and understanding its role in chemical reactions. This guide will break down the concept into easy-to-understand sections, supported by reliable data and examples.
By the end of this article, you will have a clear understanding of how to calculate the molar mass of CaCO3, why it is important, and how it is applied in real-world scenarios. Whether you are a student, educator, or professional, this guide will provide you with the knowledge you need to master this fundamental concept.
Read also:Andie Elle The Rising Star In The Music Industry
Table of Contents
- What is Molar Mass?
- Calcium Carbonate (CaCO3): An Overview
- How to Calculate the Molar Mass of CaCO3
- Applications of Calcium Carbonate in Daily Life
- Stoichiometry and the Role of CaCO3
- Environmental Significance of CaCO3
- Industrial Uses of Calcium Carbonate
- Common Misconceptions About Molar Mass
- Practical Examples of Molar Mass Calculations
- Conclusion: Why Molar Mass Matters
What is Molar Mass?
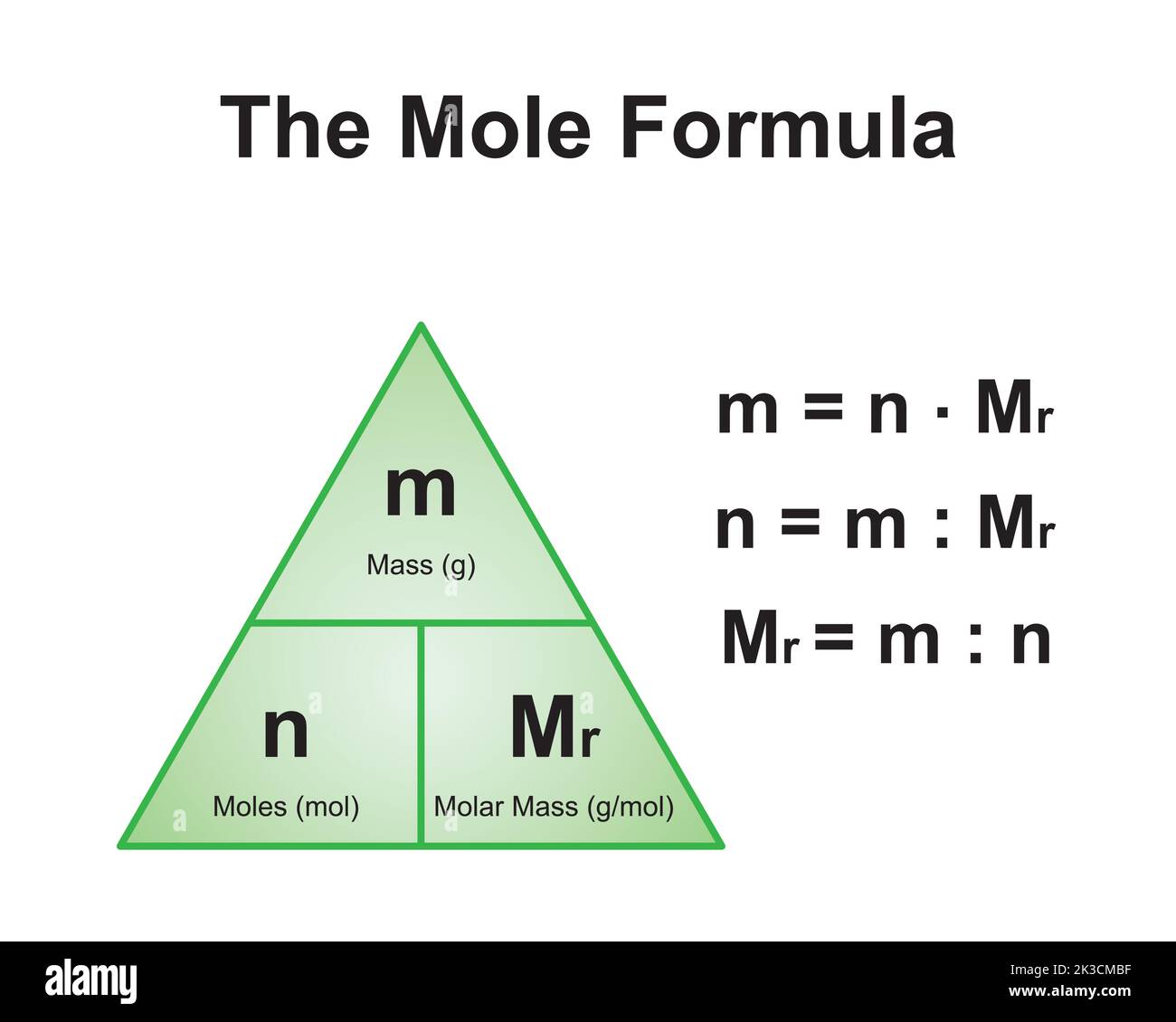
Molar mass is a fundamental concept in chemistry that refers to the mass of one mole of a substance. It is expressed in grams per mole (g/mol) and is calculated by summing the atomic masses of all the atoms in a chemical formula. For example, the molar mass of water (H2O) is approximately 18 g/mol, as it consists of two hydrogen atoms (1 g/mol each) and one oxygen atom (16 g/mol).
Understanding molar mass is crucial for performing accurate chemical calculations. It allows chemists to determine the amount of a substance needed for a reaction, predict reaction outcomes, and measure the concentration of solutions. In the case of CaCO3, knowing its molar mass is essential for applications ranging from laboratory experiments to large-scale industrial processes.
Calcium Carbonate (CaCO3): An Overview
Calcium carbonate (CaCO3) is a chemical compound composed of calcium (Ca), carbon (C), and oxygen (O). It is found in nature as minerals like calcite and aragonite and is a primary component of limestone, marble, and chalk. Below is a table summarizing the key properties of CaCO3:
| Property | Value |
|---|---|
| Chemical Formula | CaCO3 |
| Molar Mass | 100.09 g/mol |
| Appearance | White powder or crystalline solid |
| Density | 2.71 g/cm³ |
| Melting Point | 825°C (decomposes) |
Calcium carbonate is widely used in industries such as construction, agriculture, and pharmaceuticals. Its ability to neutralize acids makes it valuable in soil treatment and antacid formulations. Additionally, it serves as a filler in paper, plastics, and paints, enhancing their properties.
How to Calculate the Molar Mass of CaCO3
To calculate the molar mass of CaCO3, you need to sum the atomic masses of its constituent elements: calcium (Ca), carbon (C), and oxygen (O). Here’s a step-by-step guide:
Step 1: Identify the Atomic Masses
- Calcium (Ca): 40.08 g/mol
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol
Step 2: Multiply by the Number of Atoms
- Calcium: 1 atom × 40.08 g/mol = 40.08 g/mol
- Carbon: 1 atom × 12.01 g/mol = 12.01 g/mol
- Oxygen: 3 atoms × 16.00 g/mol = 48.00 g/mol
Step 3: Add the Values
Total molar mass = 40.08 + 12.01 + 48.00 = 100.09 g/mol
Read also:Telegram Somali Wasmo Exploring The Cultural And Social Impact Of Somali Music And Poetry
Thus, the molar mass of CaCO3 is approximately 100.09 g/mol.
Applications of Calcium Carbonate in Daily Life
Calcium carbonate has numerous applications in various fields. Below are some of its most common uses:
- Construction: Used as a raw material in cement and lime production.
- Agriculture: Acts as a soil conditioner to neutralize acidic soils.
- Pharmaceuticals: Found in antacids and dietary supplements.
- Paper Industry: Serves as a filler to improve paper quality and reduce production costs.
- Food Industry: Used as a food additive (E170) for its anticaking and pH-regulating properties.
Stoichiometry and the Role of CaCO3
Stoichiometry is the study of quantitative relationships in chemical reactions. The molar mass of CaCO3 is essential for performing stoichiometric calculations. For example, if you want to determine how much carbon dioxide (CO2) is produced when CaCO3 reacts with hydrochloric acid (HCl), you need to know the molar mass of CaCO3.
Reaction: CaCO3 + 2HCl → CaCl2 + H2O + CO2
Using the molar mass of CaCO3 (100.09 g/mol), you can calculate the amount of CO2 produced from a given quantity of CaCO3. This type of calculation is vital in industries such as mining, where precise measurements are required.
Environmental Significance of CaCO3
Calcium carbonate plays a critical role in the environment. It is a primary component of marine organisms like corals and mollusks, which use it to build their shells and skeletons. Additionally, CaCO3 helps regulate the Earth's carbon cycle by acting as a carbon sink.
However, environmental factors such as ocean acidification can impact the formation of CaCO3 structures. Increased CO2 levels in the atmosphere lead to higher acidity in oceans, making it harder for marine organisms to produce calcium carbonate. This underscores the importance of understanding and preserving natural CaCO3 resources.
Industrial Uses of Calcium Carbonate
Calcium carbonate is a versatile compound with widespread industrial applications. Some of its key uses include:
- Cement Production: Acts as a primary raw material in the manufacture of cement.
- Plastics and Rubber: Enhances the durability and strength of these materials.
- Paints and Coatings: Improves opacity and reduces costs.
- Water Treatment: Used to soften water by removing excess calcium and magnesium ions.
These applications highlight the compound's importance in modern industries and its contribution to economic growth.
Common Misconceptions About Molar Mass
Despite its importance, there are several misconceptions about molar mass. One common misunderstanding is that molar mass and molecular weight are interchangeable terms. While they are related, molecular weight refers to the mass of a single molecule, whereas molar mass pertains to one mole of a substance.
Another misconception is that molar mass calculations are only relevant for complex compounds. In reality, even simple substances like water require molar mass calculations for accurate measurements in experiments and industrial processes.
Practical Examples of Molar Mass Calculations
Here are two practical examples to illustrate the importance of molar mass calculations:
Example 1: Determining Reaction Yield
Suppose you have 50 grams of CaCO3 and want to determine how much CO2 will be produced in the reaction with HCl. Using the molar mass of CaCO3 (100.09 g/mol), you can calculate the number of moles and then the mass of CO2 produced.
Example 2: Preparing a Solution
To prepare a 0.1 M solution of CaCO3, you need to dissolve a specific amount of the compound in water. Knowing its molar mass allows you to calculate the exact mass required for the desired concentration.
Conclusion: Why Molar Mass Matters
The molar mass of CaCO3 is a fundamental concept that underpins many scientific and industrial processes. From understanding its role in environmental systems to applying it in laboratory experiments, mastering this concept is essential for anyone working in chemistry or related fields.
We hope this guide has provided you with a clear understanding of how to calculate and apply the molar mass of CaCO3. If you found this article helpful, feel free to share it with others or leave a comment below. For more informative content, explore our other articles on chemistry and science!