Understanding the molar mass of CaCO3 is crucial for students, researchers, and professionals in chemistry and related fields. Calcium carbonate, commonly known as CaCO3, is a fundamental compound used in industries ranging from pharmaceuticals to construction. Its molar mass is not only a key concept in chemistry but also plays a significant role in real-life applications, such as determining the dosage of calcium supplements or calculating the amount of limestone needed for industrial processes. This article will guide you through everything you need to know about the molar mass of CaCO3, from its calculation to its practical uses.
Calcium carbonate is a naturally occurring compound found in rocks, shells, and even in our daily lives in the form of chalk or limestone. Its chemical formula, CaCO3, indicates that it consists of one calcium (Ca) atom, one carbon (C) atom, and three oxygen (O) atoms. Understanding its molar mass is essential for various chemical reactions and applications. In this article, we will delve into the detailed calculation of its molar mass, explore its significance, and provide practical examples to help you grasp this concept thoroughly.
By the end of this article, you will not only understand how to calculate the molar mass of CaCO3 but also appreciate its importance in various industries and scientific studies. Whether you're a student preparing for exams or a professional working in a chemistry-related field, this guide will equip you with the knowledge you need to confidently handle CaCO3-related calculations and applications.
Read also:Alice Cottonsox The Rising Star In The World Of Art And Design
Table of Contents
- What is CaCO3?
- Why Molar Mass Matters
- How to Calculate the Molar Mass of CaCO3
- Applications of CaCO3 in Daily Life
- Industrial Uses of CaCO3
- Environmental Impact of CaCO3
- Common Mistakes in Calculating Molar Mass
- Practical Examples of Molar Mass Calculations
- Tools and Resources for Molar Mass Calculations
- Conclusion and Call to Action
What is CaCO3?
Calcium carbonate, or CaCO3, is a chemical compound that is abundant in nature. It is found in rocks such as limestone, marble, and chalk, and is also a key component of shells and pearls. Chemically, it consists of one calcium (Ca) atom, one carbon (C) atom, and three oxygen (O) atoms. Its molecular structure makes it an essential compound in various scientific and industrial applications.
Here are some key properties of CaCO3:
- Chemical Formula: CaCO3
- Molar Mass: Approximately 100.09 g/mol
- Appearance: White powder or crystalline solid
- Solubility: Insoluble in water but soluble in acids
CaCO3 is widely used in industries such as construction, pharmaceuticals, and agriculture. Its versatility and abundance make it a critical compound in both scientific research and everyday applications.
Why Molar Mass Matters
The molar mass of a compound is the mass of one mole of that substance, expressed in grams per mole (g/mol). For CaCO3, understanding its molar mass is essential for several reasons:
Role in Chemical Reactions
In chemical reactions, the molar mass of CaCO3 helps determine the stoichiometry of the reaction. For example, when CaCO3 reacts with hydrochloric acid (HCl), the amount of carbon dioxide (CO2) produced depends on the molar mass of CaCO3. This information is crucial for predicting reaction outcomes and optimizing processes.
Applications in Pharmaceuticals
CaCO3 is a common ingredient in antacids and calcium supplements. Knowing its molar mass ensures accurate dosing, which is critical for patient safety and treatment efficacy. Errors in molar mass calculations can lead to incorrect dosages, which may have serious health implications.
Read also:Movierulz Ui The Ultimate Guide To Streaming Movies Online Safely
Industrial Importance
In industries such as construction and agriculture, CaCO3 is used in large quantities. Accurate molar mass calculations help in determining the amount of material needed for specific applications, such as producing cement or neutralizing acidic soils.
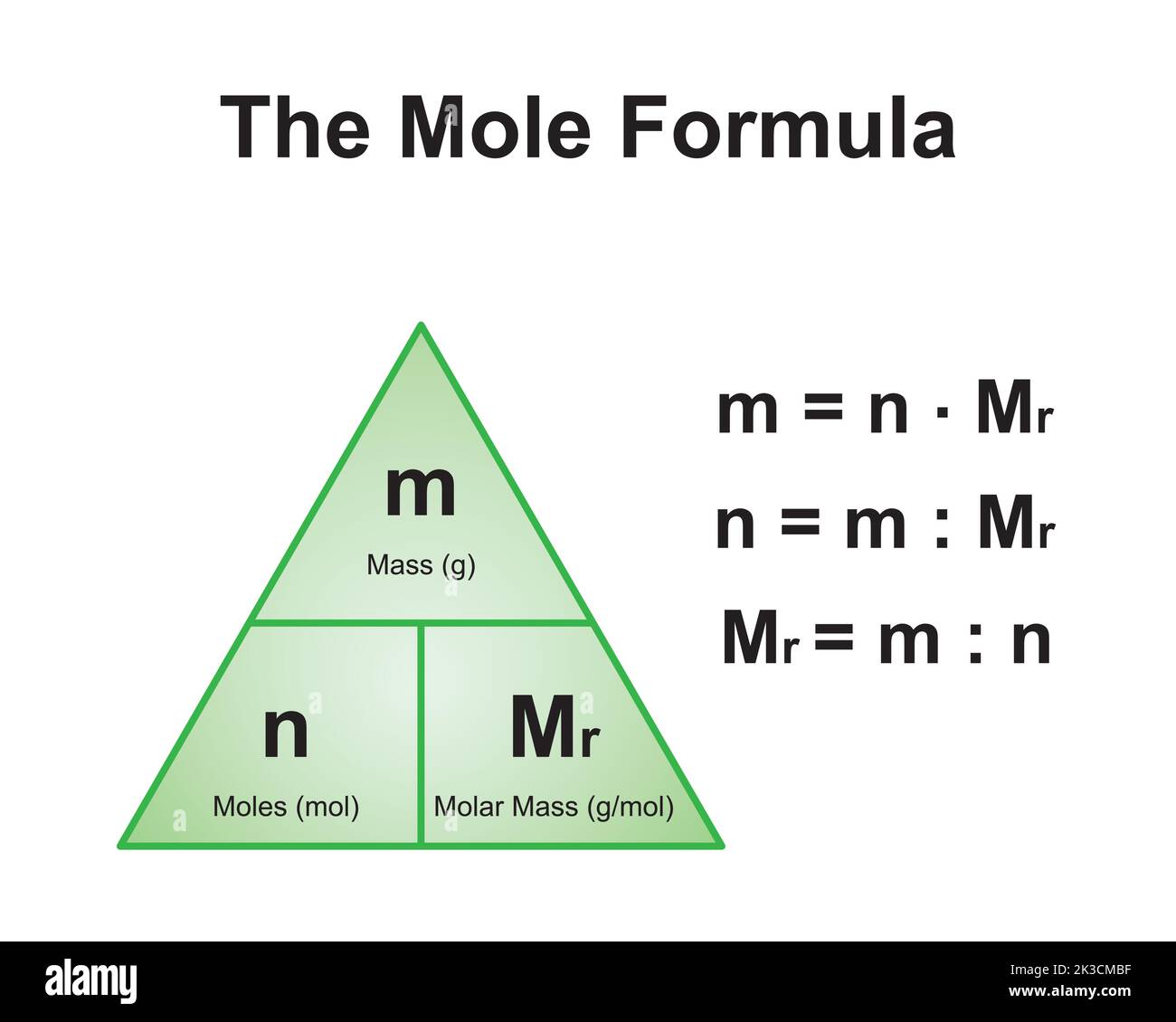
How to Calculate the Molar Mass of CaCO3
To calculate the molar mass of CaCO3, you need to sum the atomic masses of all the atoms in its chemical formula. Here's a step-by-step guide:
Step 1: Identify the Atomic Masses
Using the periodic table, find the atomic masses of calcium (Ca), carbon (C), and oxygen (O):
- Calcium (Ca): 40.08 g/mol
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol
Step 2: Multiply by the Number of Atoms
CaCO3 consists of one calcium atom, one carbon atom, and three oxygen atoms. Multiply the atomic masses by the number of atoms:
- Calcium: 1 × 40.08 = 40.08 g/mol
- Carbon: 1 × 12.01 = 12.01 g/mol
- Oxygen: 3 × 16.00 = 48.00 g/mol
Step 3: Add the Results
Sum the values obtained in Step 2:
40.08 + 12.01 + 48.00 = 100.09 g/mol
Thus, the molar mass of CaCO3 is approximately 100.09 g/mol.
Applications of CaCO3 in Daily Life
CaCO3 has numerous applications in everyday life. Below are some examples:
- Construction: Used as a raw material in the production of cement and lime.
- Pharmaceuticals: Acts as a calcium supplement and antacid.
- Agriculture: Used to neutralize acidic soils and provide essential nutrients to plants.
- Food Industry: Serves as a food additive (E170) for its anti-caking and pH-regulating properties.
Industrial Uses of CaCO3
CaCO3 is a cornerstone of many industrial processes. Its uses include:
Construction Industry
In the construction industry, CaCO3 is used to produce cement, lime, and marble. Its high purity and abundance make it a cost-effective material for large-scale projects.
Paper Manufacturing
CaCO3 is used as a filler in paper production, improving the paper's brightness and opacity. It also reduces production costs by replacing more expensive materials.
Plastics and Rubber
In the plastics and rubber industries, CaCO3 is used as a filler to enhance the mechanical properties of the final product. It also improves durability and reduces material costs.
Environmental Impact of CaCO3
While CaCO3 is widely used, its extraction and processing can have environmental consequences. Mining limestone, a primary source of CaCO3, can lead to habitat destruction and increased carbon emissions. However, CaCO3 also plays a positive role in environmental applications, such as:
- Carbon Sequestration: CaCO3 can be used to capture and store carbon dioxide, reducing greenhouse gas emissions.
- Water Treatment: It helps neutralize acidic water and remove impurities.
Common Mistakes in Calculating Molar Mass
When calculating the molar mass of CaCO3, students and professionals often make the following mistakes:
- Incorrect Atomic Masses: Using outdated or incorrect values from the periodic table.
- Omitting Atoms: Forgetting to account for all atoms in the chemical formula.
- Rounding Errors: Rounding atomic masses too early in the calculation process.
Practical Examples of Molar Mass Calculations
Let's explore a practical example of using the molar mass of CaCO3:
Example: Neutralizing Acidic Soil
To neutralize acidic soil, farmers often use CaCO3. If a farmer needs to neutralize 1 mole of acid, how much CaCO3 is required?
Since 1 mole of CaCO3 neutralizes 2 moles of acid, the farmer would need 0.5 moles of CaCO3. Using the molar mass of CaCO3 (100.09 g/mol), the required amount is:
0.5 × 100.09 = 50.045 grams
Tools and Resources for Molar Mass Calculations
Several online tools and resources can help you calculate the molar mass of CaCO3 and other compounds:
- Periodic Table: A reliable periodic table is essential for accurate atomic mass values.
- Online Calculators: Websites like ChemSpider and PubChem offer free molar mass calculators.
- Textbooks and Manuals: Chemistry textbooks often include detailed explanations and examples.
Conclusion and Call to Action
In conclusion, understanding the molar mass of CaCO3 is vital for various applications, from chemical reactions to industrial processes. By mastering its calculation and significance, you can confidently tackle problems and contribute to advancements in science and industry.
We encourage you to practice calculating the molar mass of other compounds and explore their applications. Share your thoughts or questions in the comments below, and don't forget to check out our other articles for more insights into chemistry and related fields. Together, let's continue learning and growing in this fascinating world of science!