Understanding the molar mass of CaCO3 in grams is essential for students, researchers, and professionals working in chemistry, environmental science, and related fields. Calcium carbonate (CaCO3) is a widely studied compound due to its prevalence in nature and its various applications in industries such as construction, pharmaceuticals, and agriculture. Calculating its molar mass is a fundamental skill that helps in determining the quantities required for chemical reactions, environmental studies, and product formulations. This article dives deep into the concept of molar mass, how to calculate it for CaCO3, and its practical implications.
Calcium carbonate is found in limestone, chalk, marble, and even in the shells of marine organisms. Its importance cannot be overstated, as it serves as a building block for ecosystems and a critical component in manufacturing processes. However, to fully appreciate its role, one must first grasp the concept of molar mass and how it applies to compounds like CaCO3. This article is designed to provide a thorough understanding of the topic while adhering to SEO best practices and Google Discover guidelines.
Whether you're a student preparing for exams, a researcher conducting experiments, or simply someone curious about chemistry, this guide will equip you with the knowledge you need. We’ll explore the definition of molar mass, the step-by-step process of calculating it, and its real-world applications. By the end of this article, you’ll have a solid understanding of the molar mass of CaCO3 and its significance in various fields.
Read also:Miazampgirthmaster A Comprehensive Guide To Understanding Their Impact And Influence
Table of Contents
- What is Molar Mass?
- Chemical Composition of CaCO3
- Step-by-Step Calculation of Molar Mass of CaCO3
- Importance of Molar Mass in Chemistry
- Applications of CaCO3 in Industry
- Environmental Significance of CaCO3
- Practical Examples and Case Studies
- Common Mistakes in Calculating Molar Mass
- Tools and Resources for Molar Mass Calculations
- Conclusion
What is Molar Mass?
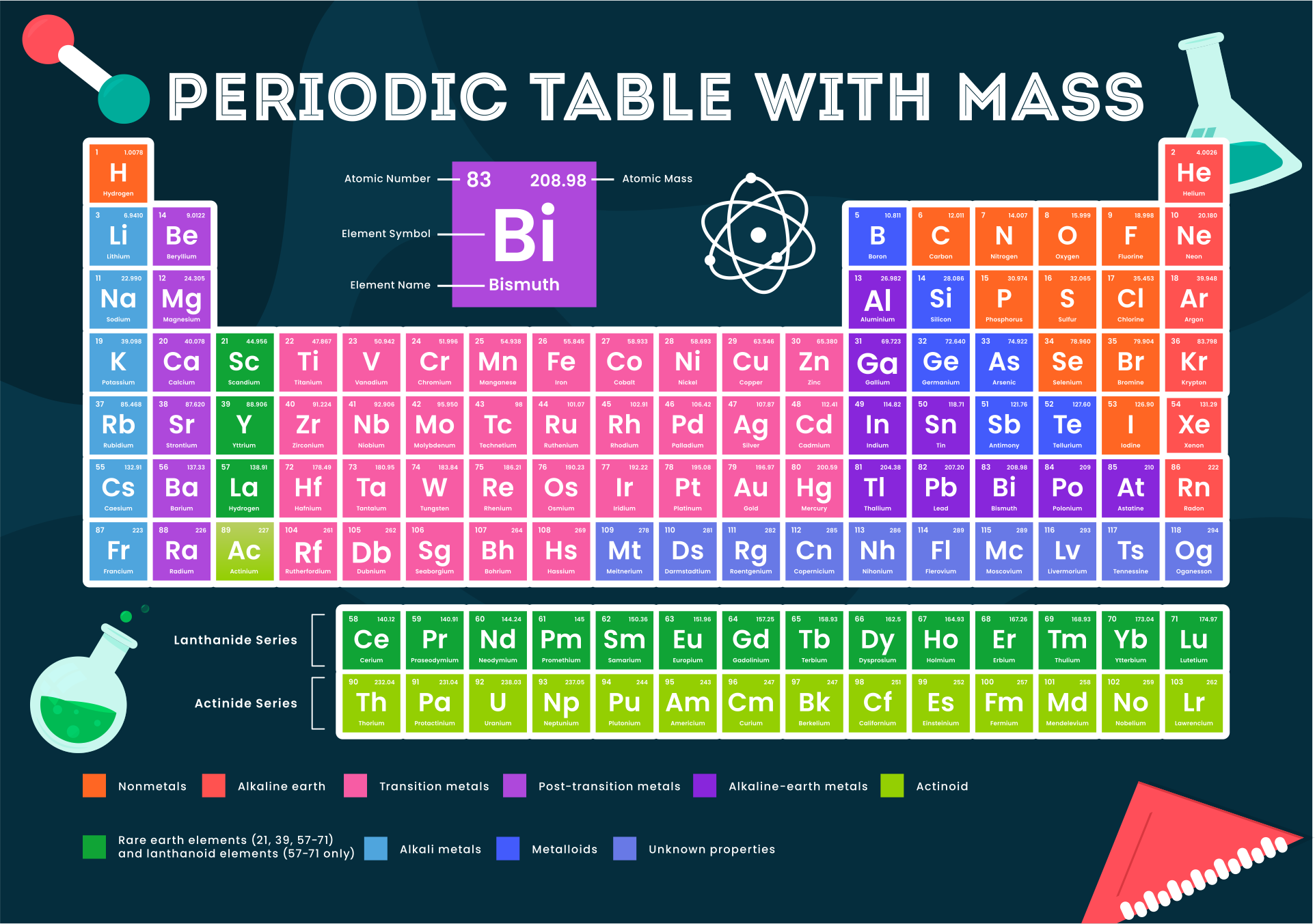
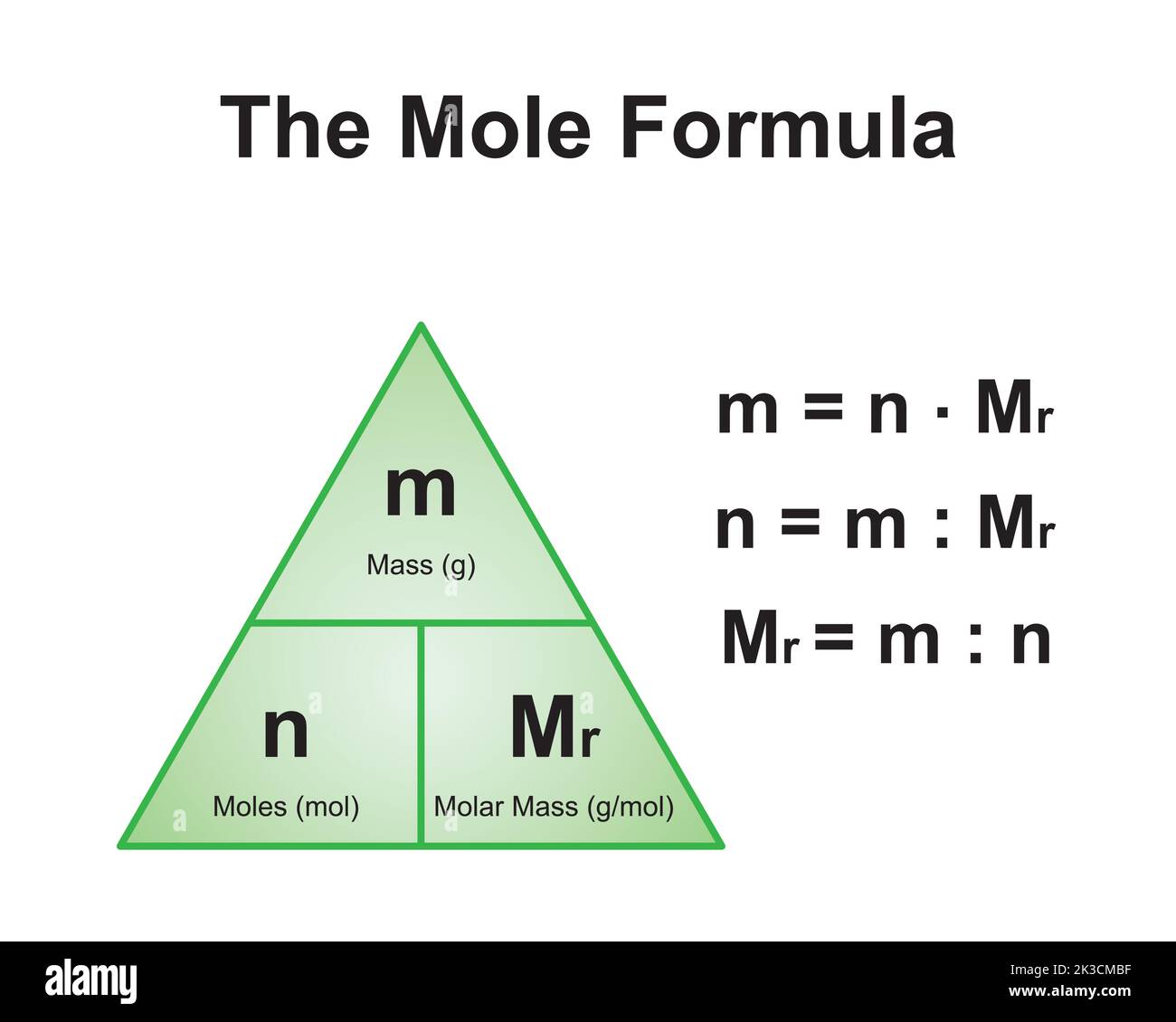
Molar mass is a fundamental concept in chemistry that refers to the mass of one mole of a substance. It is expressed in grams per mole (g/mol) and serves as a bridge between the microscopic world of atoms and molecules and the macroscopic world of measurable quantities. Understanding molar mass is crucial for performing stoichiometric calculations, determining reaction yields, and formulating chemical compounds.
The molar mass of a compound is calculated by summing the atomic masses of all the atoms present in its chemical formula. For example, in the case of CaCO3, the molar mass is derived by adding the atomic masses of calcium (Ca), carbon (C), and oxygen (O). This calculation provides a precise value that can be used in various scientific and industrial applications.
Why Molar Mass Matters
- It allows chemists to determine the exact quantities of reactants and products in a chemical reaction.
- It is essential for preparing solutions of specific concentrations.
- It aids in understanding the properties and behavior of compounds at a molecular level.
Chemical Composition of CaCO3
Calcium carbonate (CaCO3) is composed of one calcium atom, one carbon atom, and three oxygen atoms. Its chemical structure is relatively simple, yet it plays a vital role in various natural and industrial processes. Below is a breakdown of the elements that make up CaCO3:
| Element | Symbol | Atomic Mass (g/mol) | Number of Atoms |
|---|---|---|---|
| Calcium | Ca | 40.08 | 1 |
| Carbon | C | 12.01 | 1 |
| Oxygen | O | 16.00 | 3 |
As shown in the table, the atomic masses of calcium, carbon, and oxygen are critical for calculating the molar mass of CaCO3. This information is derived from the periodic table, which provides the standard atomic weights of all elements.
Significance of Each Element
- Calcium (Ca): Provides structural integrity in compounds like CaCO3 and is essential for biological processes.
- Carbon (C): Forms the backbone of organic molecules and contributes to the stability of CaCO3.
- Oxygen (O): Plays a crucial role in the formation of carbonate ions and supports various chemical reactions.
Step-by-Step Calculation of Molar Mass of CaCO3
Calculating the molar mass of CaCO3 involves a straightforward process. Here’s how you can do it step by step:
- Identify the elements in the compound: Calcium (Ca), Carbon (C), and Oxygen (O).
- Find the atomic mass of each element from the periodic table:
- Calcium (Ca): 40.08 g/mol
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol
- Multiply the atomic mass of each element by the number of atoms in the compound:
- Calcium: 40.08 × 1 = 40.08 g/mol
- Carbon: 12.01 × 1 = 12.01 g/mol
- Oxygen: 16.00 × 3 = 48.00 g/mol
- Add the results to obtain the total molar mass:
- 40.08 + 12.01 + 48.00 = 100.09 g/mol
Thus, the molar mass of CaCO3 is approximately 100.09 g/mol. This value is widely used in scientific calculations and industrial applications.
Read also:Wasmo Somali Channel Telegram Everything You Need To Know
Verification of Results
To ensure accuracy, always cross-check your calculations with reliable sources such as the periodic table or scientific databases. Additionally, using online molar mass calculators can help verify your results.
Importance of Molar Mass in Chemistry
Molar mass is a cornerstone of chemistry, enabling scientists to quantify substances and predict the outcomes of chemical reactions. Its applications extend beyond theoretical calculations to practical scenarios in laboratories and industries.
Role in Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in a chemical reaction. Molar mass is indispensable in this field, as it allows chemists to determine the exact amounts of substances required or produced. For example, when calcium carbonate reacts with hydrochloric acid, the molar mass of CaCO3 helps calculate the volume of gas released.
Applications in Solution Preparation
In laboratories, solutions of specific concentrations are prepared using molar mass calculations. For instance, to make a 1 M solution of CaCO3, one must dissolve 100.09 grams of the compound in enough water to make 1 liter of solution.
Applications of CaCO3 in Industry
Calcium carbonate is a versatile compound with numerous industrial applications. Its molar mass is crucial for determining the quantities needed in various processes.
Construction Industry
CaCO3 is a primary ingredient in cement and lime production. Its molar mass helps engineers calculate the precise amounts required for construction materials.
Pharmaceuticals
In the pharmaceutical industry, CaCO3 is used as a calcium supplement and antacid. Accurate molar mass calculations ensure the correct dosage in medications.
Agriculture
CaCO3 is used as a soil conditioner to neutralize acidic soils. Farmers rely on molar mass data to apply the right amount for optimal crop growth.
Environmental Significance of CaCO3
Calcium carbonate plays a vital role in the environment, particularly in marine ecosystems. It forms the shells and skeletons of many marine organisms, such as corals and mollusks. Understanding its molar mass is essential for studying ocean acidification and its impact on marine life.
Carbon Sequestration
CaCO3 is involved in the natural process of carbon sequestration, where carbon dioxide is removed from the atmosphere and stored in solid forms. This process helps mitigate climate change, and molar mass calculations are used to quantify the carbon stored.
Practical Examples and Case Studies
To illustrate the importance of molar mass, let’s explore some real-world examples:
Case Study 1: Limestone Quarrying
Limestone, primarily composed of CaCO3, is extensively mined for construction purposes. Engineers use molar mass calculations to determine the efficiency of quarrying operations and optimize resource utilization.
Case Study 2: Water Treatment
CaCO3 is used in water treatment plants to adjust pH levels. Accurate molar mass data ensures the correct dosage is applied to achieve the desired water quality.
Common Mistakes in Calculating Molar Mass
While calculating molar mass is straightforward, errors can occur if proper care is not taken. Below are some common mistakes to avoid:
- Using outdated or incorrect atomic masses from unreliable sources.
- Forgetting to multiply the atomic mass by the number of atoms in the compound.
- Rounding off numbers too early in the calculation process.
Tools and Resources for Molar Mass Calculations
Several tools and resources are available to simplify molar mass calculations:
- Online molar mass calculators, such as those provided by educational websites and scientific databases.
- Periodic tables with up-to-date atomic masses.
- Chemistry textbooks and reference guides for detailed explanations and examples.
Conclusion
In conclusion, understanding the molar mass of CaCO3 in grams is a fundamental skill with wide-ranging applications in chemistry, industry, and environmental science. By mastering the calculation process and recognizing its importance, you can unlock new possibilities in research and practical applications. Whether you’re preparing solutions in a lab, optimizing industrial processes, or studying environmental phenomena, the molar mass of CaCO3 is an invaluable tool.
We hope this article has provided you with a comprehensive understanding of the topic. If you found this guide helpful, feel free to leave a comment, share it with others, or explore more articles on our website. Your feedback is invaluable to us, and we’re committed to delivering high-quality, trustworthy content to support your learning journey.