Calcium carbonate (CaCO3) is a chemical compound that plays a vital role in various industries and biological processes. Whether you're a student studying chemistry, a professional working in manufacturing, or simply someone curious about the science behind everyday materials, understanding the molar mass of CaCO3 is essential. The molar mass of CaCO3 is a fundamental concept in chemistry that helps us quantify the amount of substance in a given sample. In this article, we will explore what CaCO3 is, why its molar mass matters, and how it is calculated.
Calcium carbonate is found naturally in rocks, shells, and even in our bodies as a component of bones and teeth. Its applications range from construction materials like limestone and marble to dietary supplements and antacids. Knowing the molar mass of CaCO3 allows scientists and engineers to perform precise calculations for chemical reactions, material production, and environmental studies. This article is designed to provide an in-depth understanding of CaCO3 and its molar mass, ensuring that readers can apply this knowledge in real-world scenarios.
In the following sections, we will break down the components of CaCO3, explain the concept of molar mass, and guide you through the calculation process step by step. We will also explore the practical applications of CaCO3, its role in health and industry, and answer frequently asked questions to address any lingering doubts. By the end of this article, you'll have a solid grasp of the molar mass of CaCO3 and its significance in various fields.
Read also:Bonn1e7hebunny The Ultimate Guide To Her Art Influence And Online Presence
Table of Contents
- What is Calcium Carbonate (CaCO3)?
- Chemical Composition of CaCO3
- What is Molar Mass? A Detailed Explanation
- How to Calculate the Molar Mass of CaCO3
- Practical Applications of CaCO3
- Environmental Impact of Calcium Carbonate
- Health Benefits and Uses of CaCO3
- Industrial Uses of Calcium Carbonate
- Frequently Asked Questions About CaCO3
- Conclusion: Why Understanding Molar Mass Matters
What is Calcium Carbonate (CaCO3)?
Calcium carbonate (CaCO3) is a naturally occurring chemical compound composed of calcium (Ca), carbon (C), and oxygen (O) atoms. It is one of the most abundant minerals on Earth, found in rocks such as limestone, chalk, and marble. CaCO3 is also present in biological structures like shells, coral, and pearls. Its versatility makes it a critical component in various industries, including construction, pharmaceuticals, and agriculture.
CaCO3 exists in several crystalline forms, the most common being calcite and aragonite. These forms differ in their crystal structure but share the same chemical formula. The compound is often used as a raw material in the production of cement, lime, and glass. Additionally, it serves as a dietary supplement to provide essential calcium for bone health and as an antacid to neutralize stomach acid.
Key Properties of Calcium Carbonate
- Chemical Formula: CaCO3
- Molar Mass: 100.09 g/mol (we'll explore this in detail later)
- Appearance: White powder or crystalline solid
- Solubility: Insoluble in water but soluble in acids
Chemical Composition of CaCO3
To understand the molar mass of CaCO3, we must first examine its chemical composition. Calcium carbonate consists of one calcium atom, one carbon atom, and three oxygen atoms. Each of these elements contributes to the overall molar mass of the compound.
The atomic masses of the elements in CaCO3 are as follows:
- Calcium (Ca): 40.08 g/mol
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol (multiplied by 3 for the three oxygen atoms)
By summing the atomic masses of these elements, we can calculate the total molar mass of CaCO3. This calculation will be explored in detail in the next section.
Importance of Chemical Composition
The chemical composition of CaCO3 determines its properties and applications. For example, the presence of calcium makes it an excellent source of dietary calcium, while the carbonate group (CO3) contributes to its ability to react with acids. Understanding the composition is crucial for industries that rely on precise chemical reactions and material formulations.
Read also:Robert Jamescolliers Wife Meet Name
What is Molar Mass? A Detailed Explanation
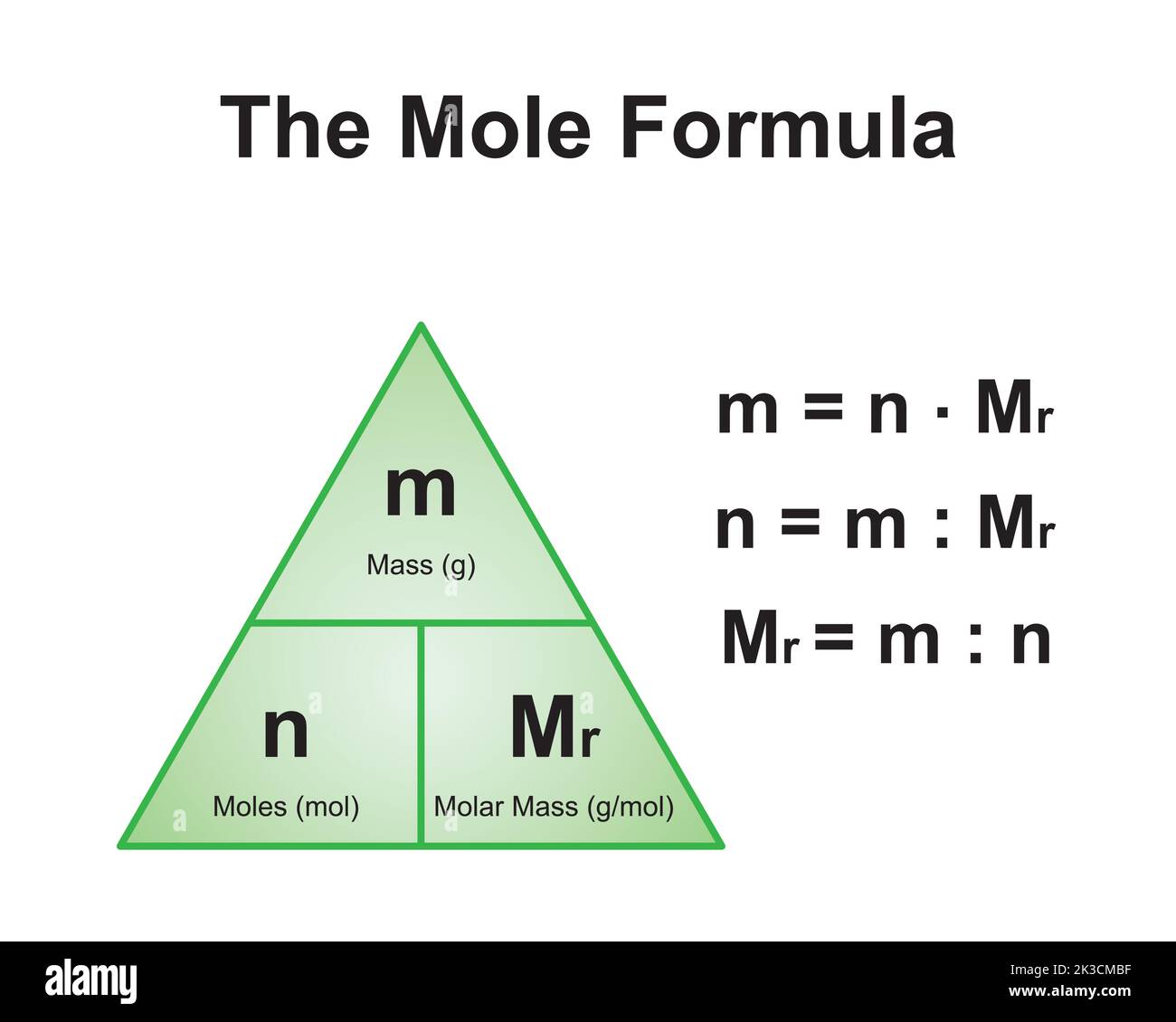
Molar mass is a fundamental concept in chemistry that refers to the mass of one mole of a substance. A mole is a unit of measurement used to quantify the amount of a substance, and it is defined as 6.022 x 10^23 particles (Avogadro's number). The molar mass of a compound is expressed in grams per mole (g/mol) and is calculated by summing the atomic masses of all the atoms in its chemical formula.
For example, the molar mass of water (H2O) is calculated by adding the atomic masses of two hydrogen atoms (2 x 1.008 g/mol) and one oxygen atom (16.00 g/mol), resulting in a total of 18.016 g/mol. Similarly, the molar mass of CaCO3 is determined by summing the atomic masses of calcium, carbon, and oxygen.
Why is Molar Mass Important?
Molar mass is essential for various reasons:
- Stoichiometry: It allows chemists to calculate the amounts of reactants and products in chemical reactions.
- Concentration: It helps determine the concentration of solutions in terms of molarity (moles per liter).
- Material Science: It is used to design materials with specific properties, such as strength or solubility.
How to Calculate the Molar Mass of CaCO3
To calculate the molar mass of CaCO3, we need to sum the atomic masses of all the atoms in its chemical formula. Here's a step-by-step breakdown:
Step 1: Identify the Atoms and Their Quantities
- Calcium (Ca): 1 atom
- Carbon (C): 1 atom
- Oxygen (O): 3 atoms
Step 2: Find the Atomic Masses
- Calcium (Ca): 40.08 g/mol
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol (multiplied by 3)
Step 3: Perform the Calculation
Molar mass of CaCO3 = (1 x 40.08) + (1 x 12.01) + (3 x 16.00)
Molar mass of CaCO3 = 40.08 + 12.01 + 48.00
Molar mass of CaCO3 = 100.09 g/mol
Final Result
The molar mass of CaCO3 is 100.09 g/mol. This value is crucial for performing accurate calculations in chemistry and related fields.
Practical Applications of CaCO3
Calcium carbonate has a wide range of applications across various industries. Its versatility stems from its chemical properties and abundance in nature. Below are some of the most common uses of CaCO3:
1. Construction Industry
- Used as a raw material in the production of cement and lime.
- Acts as a filler in paints, coatings, and adhesives to improve texture and durability.
2. Pharmaceuticals
- Serves as a calcium supplement in dietary products.
- Used as an antacid to neutralize stomach acid and relieve heartburn.
3. Agriculture
- Applied as a soil conditioner to adjust pH levels and improve crop yield.
- Used as a feed additive for livestock to provide essential calcium.
4. Environmental Applications
- Utilized in water treatment processes to remove impurities and adjust pH.
- Employed in flue gas desulfurization to reduce sulfur dioxide emissions.
Environmental Impact of Calcium Carbonate
While calcium carbonate is a naturally occurring compound, its extraction and use can have environmental implications. Mining limestone, a primary source of CaCO3, can lead to habitat destruction and increased carbon emissions. However, CaCO3 also plays a positive role in environmental sustainability.
Positive Contributions
- Used in carbon capture technologies to mitigate greenhouse gas emissions.
- Helps neutralize acidic soils and water bodies, promoting biodiversity.
Challenges
- Over-mining can deplete natural resources and disrupt ecosystems.
- Improper disposal of CaCO3-based products may contribute to pollution.
Health Benefits and Uses of CaCO3
Calcium carbonate is widely used in the healthcare industry due to its health benefits. It is a key ingredient in dietary supplements and medications, providing essential nutrients and therapeutic effects.
1. Bone Health
Calcium is a critical mineral for maintaining strong bones and teeth. CaCO3 supplements are often recommended for individuals at risk of osteoporosis or calcium deficiency.
2. Antacid Properties
CaCO3 reacts with stomach acid (hydrochloric acid) to form calcium chloride, water, and carbon dioxide. This reaction helps neutralize excess acid and relieve symptoms of heartburn and indigestion.
3. Dental Care
Calcium carbonate is used in toothpaste to provide a mild abrasive action, helping to remove plaque and stains without damaging tooth enamel.
Industrial Uses of Calcium Carbonate
Calcium carbonate is a versatile compound with numerous industrial applications. Its unique properties make it an indispensable material in manufacturing and production processes.
1. Paper Industry
CaCO3 is used as a filler and coating pigment in paper production, improving brightness, opacity, and printability.
2. Plastics and Rubber
It acts as a filler in plastics and rubber to enhance strength, durability, and cost-effectiveness.
3. Food Industry
CaCO3 is used as a food additive (E170) to fortify products with calcium and as a leavening agent in baking.
Frequently Asked Questions About CaCO3
1. What is the molar mass of CaCO3?
The molar mass of CaCO3 is 100.09 g/mol.
2. Is CaCO3 soluble in water?
Calcium carbonate is insoluble in water but dissolves in acidic solutions.
3. What are the health benefits of CaCO3?
CaCO3 supports bone health, acts as an antacid, and is used in dental care products.
4. Where is CaCO3 found naturally?
CaCO3 is found in rocks like limestone and marble, as well as in shells, coral, and pearls.
5. Can CaCO3 be used in construction?
Yes, it is a key ingredient in cement, lime, and other building materials.