Table of Contents
Introduction
CH2O molecular geometry is a fascinating topic in chemistry that helps us understand the structure and behavior of formaldehyde, a common organic compound. Understanding molecular geometry is essential for predicting how molecules interact in chemical reactions and how their shapes influence their properties. In this article, we will explore the molecular geometry of CH2O, its bond angles, hybridization, and applications, ensuring you gain a comprehensive understanding of this important concept.
Formaldehyde (CH2O) is a simple yet crucial molecule in various industries, including pharmaceuticals, resins, and disinfectants. Its molecular geometry plays a significant role in determining its chemical reactivity and physical properties. By delving into the intricacies of CH2O's molecular structure, we can better appreciate its significance in both theoretical and practical chemistry.
This article will guide you through the basics of molecular geometry, the principles of VSEPR theory, and how these concepts apply to CH2O. We will also discuss the importance of understanding molecular geometry in real-world applications and how it impacts our daily lives.
Read also:Monalitaxo Unveiling The Rising Star In The Digital World
What is CH2O?
CH2O, commonly known as formaldehyde, is a naturally occurring organic compound with the chemical formula CH2O. It is the simplest aldehyde, characterized by a central carbon atom double-bonded to an oxygen atom and single-bonded to two hydrogen atoms. Formaldehyde is a colorless gas with a pungent odor and is widely used in various industries due to its versatile chemical properties.
Here are some key facts about CH2O:
- Chemical Formula: CH2O
- Molar Mass: 30.03 g/mol
- Appearance: Colorless gas
- Odor: Strong, pungent
- Boiling Point: -19°C (at standard pressure)
Formaldehyde is used in the production of resins, plastics, and disinfectants. It is also a critical component in the synthesis of various chemicals, including adhesives, coatings, and textiles. Understanding its molecular geometry helps scientists predict its reactivity and optimize its use in industrial processes.
Molecular Geometry Basics
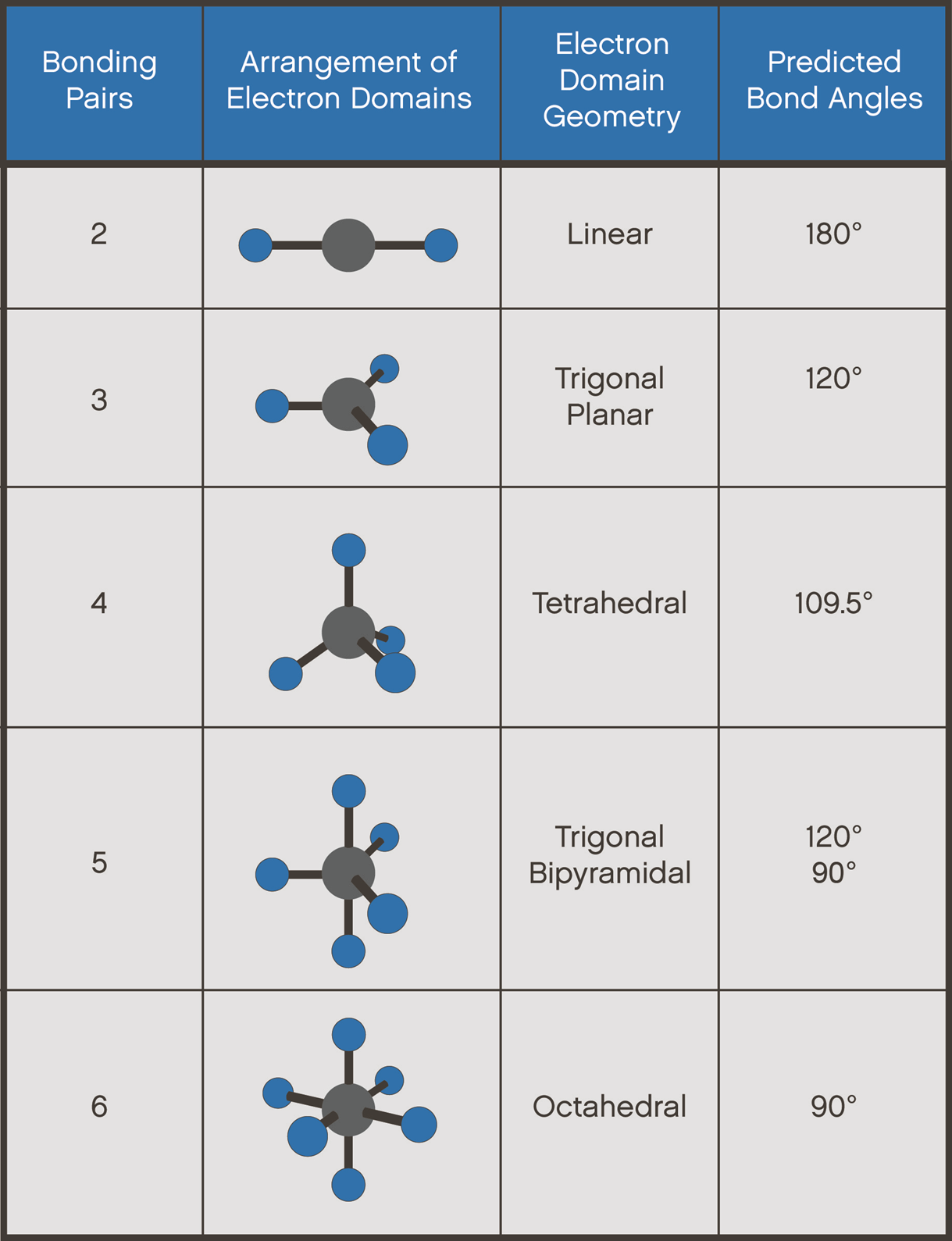
Molecular geometry refers to the arrangement of atoms in a molecule and how they are spatially positioned relative to one another. It is a fundamental concept in chemistry that helps explain the physical and chemical properties of molecules. The geometry of a molecule is determined by the number of electron pairs around the central atom, including both bonding and non-bonding pairs.
Why is molecular geometry important?
- It influences the polarity of molecules, which affects their solubility and reactivity.
- It determines the shape of molecules, impacting how they interact with other substances.
- It plays a crucial role in enzyme-substrate interactions and biological processes.
Understanding molecular geometry requires knowledge of concepts like valence electrons, electron pair repulsion, and hybridization. These principles form the foundation for predicting and analyzing the shapes of molecules, including CH2O.
Read also:Discover The Enchanting World Of Soundra Blust A Comprehensive Guide
Determining CH2O Molecular Geometry
To determine the molecular geometry of CH2O, we must first analyze its Lewis structure. The central carbon atom in CH2O forms a double bond with oxygen and single bonds with two hydrogen atoms. This arrangement results in a trigonal planar geometry, where the atoms are positioned in a flat, triangular shape.
Steps to determine CH2O geometry:
- Draw the Lewis structure of CH2O.
- Count the number of electron pairs around the central carbon atom.
- Apply VSEPR theory to predict the molecular shape.
- Verify the bond angles and confirm the geometry.
The trigonal planar geometry of CH2O ensures that the molecule has minimal electron pair repulsion, leading to a stable and efficient structure.
VSEPR Theory and Its Role in CH2O Geometry
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a model used to predict the geometry of molecules based on the repulsion between electron pairs. According to VSEPR, electron pairs around a central atom arrange themselves to minimize repulsion, resulting in specific molecular shapes.
In the case of CH2O, the central carbon atom has three regions of electron density: one double bond with oxygen and two single bonds with hydrogen atoms. This arrangement leads to a trigonal planar geometry, with bond angles of approximately 120°.
VSEPR and Bond Angles
The bond angles in CH2O are determined by the repulsion between electron pairs. The double bond between carbon and oxygen exerts slightly more repulsion than the single bonds with hydrogen, but the overall geometry remains trigonal planar.
Bond Angles and Shape of CH2O
The bond angles in CH2O are approximately 120°, consistent with its trigonal planar geometry. This angle is a result of the equal repulsion between the three electron pairs around the central carbon atom.
Key characteristics of CH2O's shape:
- Flat, triangular arrangement of atoms.
- Central carbon atom at the apex of the triangle.
- Double bond with oxygen and single bonds with hydrogen atoms.
The trigonal planar shape of CH2O ensures that the molecule is stable and minimizes electron pair repulsion, making it highly reactive in various chemical processes.
Hybridization in CH2O
Hybridization is the process of mixing atomic orbitals to form new hybrid orbitals that are suitable for bonding. In CH2O, the central carbon atom undergoes sp² hybridization, resulting in three sp² hybrid orbitals and one unhybridized p orbital.
Key points about hybridization in CH2O:
- Carbon's sp² hybridization allows it to form three sigma bonds (two with hydrogen and one with oxygen).
- The unhybridized p orbital of carbon forms a pi bond with oxygen, resulting in the double bond.
- The trigonal planar geometry is a direct result of sp² hybridization.
Impact of Hybridization on Geometry
The sp² hybridization of carbon in CH2O ensures that the molecule has a flat, planar structure with bond angles of approximately 120°. This hybridization is crucial for understanding the molecule's reactivity and stability.
Applications of CH2O
Formaldehyde (CH2O) has a wide range of applications in various industries due to its unique chemical properties and molecular geometry. Some of the key applications include:
- Production of resins and adhesives.
- Manufacture of plastics and textiles.
- Use as a disinfectant and preservative.
- Component in the synthesis of pharmaceuticals.
The trigonal planar geometry of CH2O contributes to its high reactivity, making it a valuable compound in industrial processes. Understanding its molecular structure helps scientists optimize its use and develop new applications.
Factors Affecting Molecular Geometry
Several factors influence the molecular geometry of a compound, including the number of electron pairs, the type of bonds, and the presence of lone pairs. In the case of CH2O, the following factors play a significant role:
- Electron pair repulsion between bonding and non-bonding pairs.
- Hybridization of the central atom.
- Type of bonds (single, double, or triple).
Understanding these factors is essential for predicting and analyzing the geometry of molecules, ensuring accurate predictions of their properties and behavior.
Importance of Understanding Molecular Geometry
Understanding molecular geometry is crucial for several reasons:
- It helps predict the physical and chemical properties of molecules.
- It aids in the design of new materials and drugs with specific properties.
- It provides insights into biological processes and enzyme-substrate interactions.
By studying the molecular geometry of CH2O, scientists can optimize its use in industrial processes and develop new applications that benefit society.
Conclusion
In this article, we have explored the molecular geometry of CH2O, its bond angles, hybridization, and applications. Understanding the trigonal planar geometry of CH2O is essential for predicting its reactivity and optimizing its use in various industries. By applying concepts like VSEPR theory and hybridization, we can gain valuable insights into the behavior of molecules and their impact on our daily lives.
We encourage you to share your thoughts in the comments section below or explore other articles on our site to deepen your understanding of molecular geometry and its applications. Your feedback and engagement help us create content that is informative, accurate, and valuable to our readers.