COCl2 molar mass is a fundamental concept in chemistry, particularly for those studying inorganic compounds and their properties. Phosgene, with the chemical formula COCl2, is a colorless gas that plays a significant role in various industrial applications, including the production of plastics and pesticides. Understanding its molar mass is crucial for performing accurate chemical calculations and ensuring safety in laboratory settings.
Calculating the molar mass of COCl2 involves determining the atomic weights of carbon, oxygen, and chlorine atoms and summing them according to the molecular formula. This process requires precision and a clear understanding of the periodic table, as slight miscalculations can lead to significant errors in chemical reactions and experiments.
In this article, we will delve deep into the concept of COCl2 molar mass, exploring its significance, calculation methods, and practical applications. Whether you're a student, researcher, or industry professional, this comprehensive guide will provide you with valuable insights and practical knowledge to enhance your understanding of this important chemical compound.
Read also:Exploring The World Of Www Masa49 Com A Comprehensive Guide
Table of Contents
What is COCl2?
COCl2, commonly known as phosgene, is a chemical compound composed of carbon, oxygen, and chlorine atoms. It exists as a colorless gas under standard conditions and has a molecular weight that can be precisely calculated using the periodic table. The compound's name "phosgene" originates from Greek, meaning "produced by light," due to its historical discovery through photochemical reactions.
Phosgene was first synthesized in 1812 by John Davy through the reaction of carbon monoxide and chlorine using sunlight as a catalyst. Despite its dangerous nature, COCl2 has found significant applications in various industries, particularly in the production of important chemical intermediates and polymers. Understanding its molecular structure and properties is essential for safe handling and effective utilization in industrial processes.
Chemical Structure of COCl2
The molecular structure of COCl2 consists of a central carbon atom double-bonded to an oxygen atom and single-bonded to two chlorine atoms. This trigonal planar arrangement gives phosgene its characteristic properties and reactivity. The bond angles and lengths are crucial parameters that affect its chemical behavior and interactions with other substances.
The Importance of Molar Mass in Chemistry
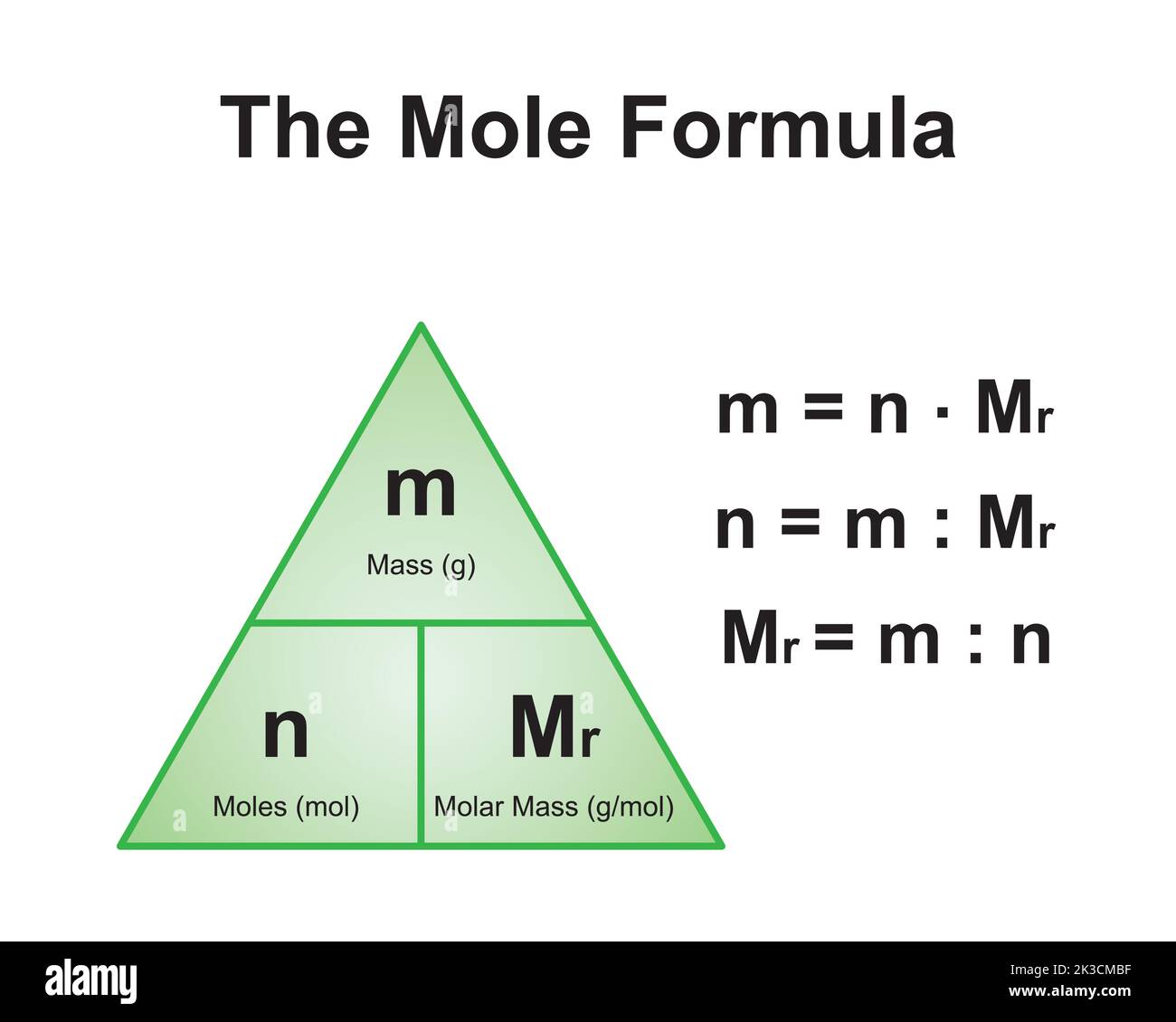
The concept of molar mass is fundamental in chemistry, serving as a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and liters. For COCl2, understanding its molar mass is particularly crucial due to its role in various chemical reactions and industrial processes. The molar mass allows chemists to convert between the number of molecules and their mass, enabling precise measurements and calculations in laboratory settings.
In practical applications, the molar mass of COCl2 is essential for determining reaction stoichiometry, calculating concentrations, and ensuring proper safety measures. When working with potentially hazardous substances like phosgene, accurate knowledge of molar mass is vital for maintaining safe working conditions and preventing accidents. Additionally, understanding molar mass helps in optimizing industrial processes and improving efficiency in chemical manufacturing.
Significance in Chemical Calculations
- Determining reaction stoichiometry and yield
- Calculating solution concentrations and dilutions
- Converting between mass and moles in chemical equations
- Estimating gas volumes using ideal gas laws
How to Calculate COCl2 Molar Mass
Calculating the molar mass of COCl2 requires precise knowledge of the atomic weights of its constituent elements. The process involves summing the atomic masses of one carbon atom, one oxygen atom, and two chlorine atoms, as indicated by the molecular formula. According to the latest IUPAC standard atomic weights, these values are:
Read also:Camilla Araujo Videos A Comprehensive Guide To Her Content And Influence
- Carbon (C): 12.011 g/mol
- Oxygen (O): 15.999 g/mol
- Chlorine (Cl): 35.45 g/mol
The calculation proceeds as follows: (1 × 12.011) + (1 × 15.999) + (2 × 35.45) = 98.91 g/mol. This precise value of COCl2 molar mass is crucial for accurate chemical calculations and experimental work.
Step-by-Step Calculation Process
- Identify the elements in the molecular formula
- Find their respective atomic weights from the periodic table
- Multiply each atomic weight by its subscript in the formula
- Sum all the calculated values
- Round the final result to appropriate significant figures
Practical Applications of COCl2
Despite its hazardous nature, COCl2 finds extensive use in various industrial applications, particularly in the production of important chemical intermediates and final products. The most significant application of phosgene is in the synthesis of isocyanates, which are crucial components in polyurethane production. These materials are widely used in manufacturing flexible and rigid foams, coatings, adhesives, and sealants.
In the pharmaceutical industry, COCl2 serves as a reagent in the production of various active pharmaceutical ingredients (APIs) and intermediates. Its reactivity makes it valuable in creating specific chemical bonds and functional groups that are difficult to achieve through other methods. Additionally, phosgene derivatives are used in the production of polycarbonate plastics, which find applications in optical media, automotive components, and electronic devices.
Industrial Uses in Detail
- Polyurethane production (TDI and MDI synthesis)
- Pharmaceutical intermediate manufacturing
- Polycarbonate plastic production
- Organic synthesis and chemical research
Safety Measures When Handling COCl2
Due to its highly toxic nature, handling COCl2 requires strict adherence to safety protocols and regulations. Phosgene is classified as an extremely hazardous substance by the Environmental Protection Agency (EPA), with an Immediately Dangerous to Life or Health (IDLH) concentration of 2 ppm. Understanding and implementing proper safety measures is crucial to prevent accidents and ensure the well-being of workers in industrial settings.
Personal protective equipment (PPE) is mandatory when working with COCl2, including full-face respirators with appropriate gas cartridges, chemical-resistant gloves, and protective clothing. Facilities handling phosgene must maintain proper ventilation systems, gas detection equipment, and emergency response protocols. Regular training and safety drills are essential to prepare workers for potential exposure scenarios.
Recommended Safety Protocols
- Use only in well-ventilated areas or fume hoods
- Implement continuous gas monitoring systems
- Maintain appropriate PPE at all times
- Develop and practice emergency response procedures
Chemical Properties of COCl2
COCl2 exhibits several distinctive chemical properties that make it valuable in industrial applications while also posing significant handling challenges. Its molecular structure, featuring a central carbon atom double-bonded to oxygen and single-bonded to two chlorine atoms, gives rise to unique reactivity patterns. The compound's trigonal planar geometry and polar bonds contribute to its chemical behavior and interaction with other substances.
Phosgene is highly reactive, particularly with nucleophiles, and readily undergoes addition reactions with alcohols, amines, and other functional groups. This reactivity is exploited in various industrial processes but also necessitates careful handling to prevent unintended reactions. The compound's boiling point is 8.3°C, and it has a vapor pressure of 1620 mmHg at 20°C, making it highly volatile under standard conditions.
Key Chemical Characteristics
- Molecular geometry: Trigonal planar
- Boiling point: 8.3°C
- Vapor pressure: 1620 mmHg at 20°C
- Reactivity with nucleophiles and functional groups
Industrial Uses of COCl2
The industrial applications of COCl2 extend across multiple sectors, with its primary use being in the production of isocyanates for polyurethane manufacturing. This versatile compound serves as a crucial intermediate in the synthesis of toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI), which are fundamental components in the production of various polyurethane products.
In the pharmaceutical industry, COCl2 plays a vital role in the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Its ability to form specific chemical bonds and functional groups makes it invaluable in creating complex molecular structures. Additionally, phosgene derivatives are extensively used in the production of polycarbonate plastics, which find applications in optical media, automotive components, and electronic devices.
Major Industrial Applications
- Polyurethane production (TDI and MDI synthesis)
- Pharmaceutical intermediate manufacturing
- Polycarbonate plastic production
- Organic synthesis and chemical research
Environmental Impact of COCl2
The environmental impact of COCl2 is a significant concern due to its high toxicity and potential for atmospheric release. When released into the environment, phosgene can persist in the atmosphere for several weeks, undergoing photochemical reactions that may produce other harmful compounds. Its high reactivity with water vapor can lead to the formation of hydrochloric acid, contributing to acid rain and environmental degradation.
Industrial facilities handling COCl2 must implement strict emission control measures and monitoring systems to minimize environmental impact. Modern production facilities utilize closed-loop systems and advanced scrubbing technologies to capture and neutralize phosgene emissions. The development of alternative, less hazardous reagents for industrial processes continues to be an area of active research, aiming to reduce the environmental footprint of phosgene-based chemistry.
Environmental Considerations
- Atmospheric persistence and photochemical reactions
- Potential for acid rain formation
- Emission control technologies
- Research into alternative reagents
Frequently Asked Questions about COCl2 Molar Mass
Understanding COCl2 molar mass often raises several common questions among students and professionals. One frequent inquiry concerns the precision of atomic weights used in calculations. The IUPAC standard atomic weights provide the most accurate values, but it's important to note that these values may have slight variations depending on the source material's isotopic composition.
Another common question relates to the significance of significant figures in molar mass calculations. When calculating COCl2 molar mass, it's crucial to maintain appropriate significant figures throughout the calculation process to ensure accuracy and precision in subsequent chemical calculations. Additionally, many wonder about the impact of temperature and pressure on molar mass measurements, though these factors primarily affect gas volume rather than molecular weight.
Common Questions and Answers
- How precise should molar mass calculations be?
- Does temperature affect COCl2 molar mass?
- Why are significant figures important in molar mass calculations?
- How does isotopic composition affect atomic weights?
Conclusion
Understanding COCl2 molar mass is essential for anyone working with this important chemical compound. From its fundamental role in chemical calculations to its practical applications in various industries, precise knowledge of phosgene's molar mass enables accurate experimentation and safe handling. Throughout this article,