Iron(II) sulfate, commonly known as FeSO4, is a chemical compound that plays a crucial role in various industries and biological processes. This compound is widely recognized for its diverse applications, ranging from agriculture to medicine. Understanding the name and properties of FeSO4 is essential for anyone working in chemistry-related fields or those simply interested in expanding their knowledge of chemical compounds. In this article, we will explore the intricacies of FeSO4 compound name, its chemical properties, and its significance in different sectors.
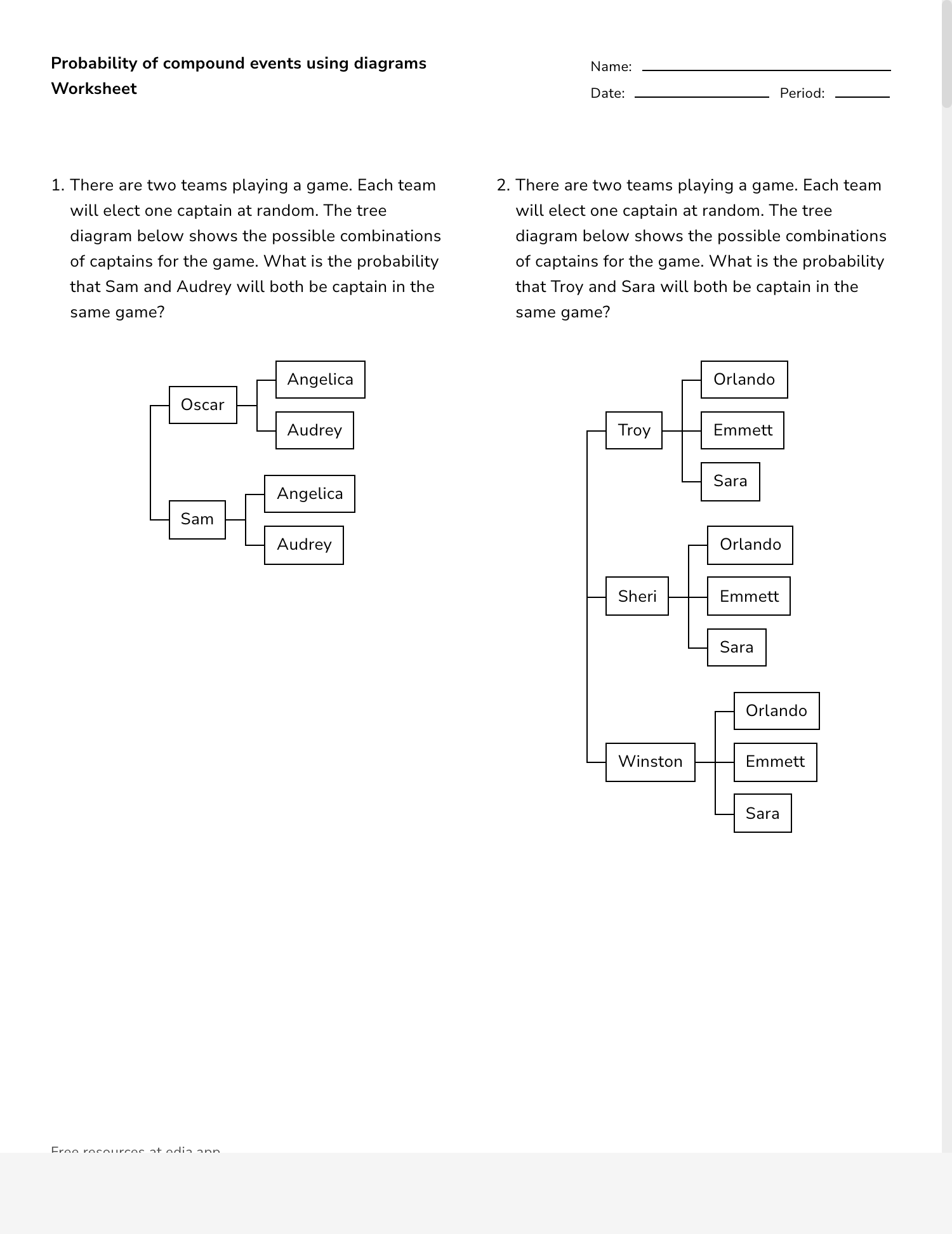
FeSO4 is an inorganic compound that consists of iron in its +2 oxidation state, sulfate ions, and water molecules in its hydrated form. This compound has been used for centuries in various applications, making it an important subject of study in chemistry. Its unique properties and versatility have made it indispensable in numerous industrial processes and biological functions. As we delve deeper into this topic, we will uncover the fascinating aspects of this compound and its impact on our daily lives.
Throughout this comprehensive guide, we will examine the chemical structure of FeSO4, its various forms, and its applications across different industries. We will also explore the safety considerations and environmental impact of this compound. By the end of this article, you will have gained valuable insights into the world of FeSO4 and its significance in modern science and industry. Whether you are a student, researcher, or simply curious about chemical compounds, this article aims to provide you with a thorough understanding of FeSO4 compound name and its various aspects.
Read also:Erika Buenfil Net Worth 2023 A Look Inside
Table of Contents
- Chemical Composition and Structure
- Understanding the Naming Convention
- Physical Properties of FeSO4
- Chemical Properties and Reactions
- Industrial Applications of FeSO4
- Biological Significance and Uses
- Safety Precautions and Handling Guidelines
- Environmental Impact and Sustainability
- Market Analysis and Future Trends
- Conclusion and Final Thoughts
Chemical Composition and Structure of FeSO4
FeSO4, or iron(II) sulfate, is composed of one iron (Fe) atom, one sulfur (S) atom, and four oxygen (O) atoms. The compound typically exists in its hydrated form, most commonly as FeSO4·7H2O, where seven water molecules are incorporated into its crystal structure. This hydrated form is often referred to as "green vitriol" due to its characteristic pale green color.
Crystal Structure and Bonding
The crystal structure of FeSO4 is monoclinic, with iron atoms occupying octahedral sites surrounded by water molecules and sulfate ions. The bonding in FeSO4 involves:
- Covalent bonds within the sulfate ion (SO4²⁻)
- Ionic bonds between the Fe²⁺ cation and the SO4²⁻ anion
- Hydrogen bonding with water molecules in the hydrated form
Isomeric Forms and Variants
FeSO4 can exist in several forms, including:
- Monohydrate (FeSO4·H2O)
- Heptahydrate (FeSO4·7H2O)
- Anhydrous form (FeSO4)
Each form exhibits slightly different properties and applications, with the heptahydrate being the most common in commercial use.
Understanding the Naming Convention of FeSO4
The systematic naming of FeSO4 follows the IUPAC (International Union of Pure and Applied Chemistry) nomenclature rules. The compound's name, iron(II) sulfate, provides crucial information about its composition and oxidation state.
Breakdown of the Chemical Name
The name "iron(II) sulfate" can be dissected as follows:
Read also:New Viral Mms Understanding The Trend And Its Impact
- "Iron" indicates the metal cation present in the compound
- "(II)" specifies the oxidation state of iron (+2)
- "Sulfate" refers to the SO4²⁻ anion
Historical Naming and Common Terms
Throughout history, FeSO4 has been known by various names:
- Green vitriol (due to its color)
- Copperas (historical term for sulfates)
- Ferrous sulfate (traditional chemical name)
These alternative names often appear in older literature and industrial contexts.
Physical Properties of FeSO4
FeSO4 exhibits distinct physical characteristics that make it easily identifiable and suitable for various applications. Understanding these properties is crucial for its proper handling and utilization.
Appearance and Solubility
The compound typically appears as:
- Blue-green crystals (heptahydrate form)
- White to off-white powder (anhydrous form)
FeSO4 demonstrates excellent solubility in water, with approximately 28.8 g dissolving in 100 mL at 25°C. It is also soluble in methanol but shows limited solubility in ethanol.
Thermal Properties
Key thermal characteristics include:
- Melting point: 64°C (heptahydrate form)
- Decomposition temperature: 480°C
- Heat of solution: -82.8 kJ/mol
Chemical Properties and Reactions of FeSO4
FeSO4 participates in numerous chemical reactions, making it a versatile compound in both laboratory and industrial settings. Understanding its chemical behavior is essential for its safe and effective use.
Oxidation and Reduction Reactions
FeSO4 is a reducing agent and readily participates in redox reactions:
- Oxidation to Fe³⁺ in the presence of air or oxidizing agents
- Reduction of other metal ions in solution
- Reaction with potassium permanganate in titration analysis
Complex Formation and Precipitation
FeSO4 can form various complexes and precipitates:
- Formation of Prussian blue with potassium ferricyanide
- Precipitation of iron hydroxide in basic solutions
- Complexation with organic ligands in analytical chemistry
Industrial Applications of FeSO4
The versatility of FeSO4 has led to its widespread use in various industrial sectors. Its applications range from water treatment to manufacturing processes.
Water Treatment and Purification
FeSO4 serves as a crucial component in:
- Coagulation and flocculation processes
- Removal of phosphate from wastewater
- Heavy metal precipitation in industrial effluents
Pigment and Dye Production
In the pigment industry, FeSO4 is used for:
- Manufacturing iron oxide pigments
- Production of inorganic colorants
- Creation of specialized coatings and paints
Biological Significance and Uses of FeSO4
FeSO4 plays a vital role in biological systems and medical applications, particularly in addressing iron deficiency-related conditions.
Medical Applications and Nutritional Supplements
FeSO4 is widely used in:
- Treatment of iron deficiency anemia
- Fortification of food products
- Manufacture of multivitamin supplements
Its bioavailability and effectiveness make it a preferred choice in medical formulations.
Agricultural Uses
In agriculture, FeSO4 serves as:
- Micronutrient fertilizer
- Corrective treatment for iron chlorosis in plants
- Soil conditioner and pH adjuster
Safety Precautions and Handling Guidelines for FeSO4
While FeSO4 is generally considered safe when handled properly, certain precautions must be observed to ensure safe usage and storage.
Personal Protective Equipment (PPE)
Recommended safety equipment includes:
- Chemical-resistant gloves
- Safety goggles or face shield
- Protective clothing
Storage and Handling Recommendations
Proper storage practices involve:
- Keeping containers tightly closed
- Storing in a cool, dry place
- Avoiding contact with incompatible materials
Environmental Impact and Sustainability of FeSO4
The environmental implications of FeSO4 usage must be carefully considered to ensure sustainable practices in its application.
Ecosystem Effects and Biodegradability
FeSO4's environmental impact includes:
- Potential for water body acidification
- Effects on aquatic organisms at high concentrations
- Natural degradation processes in soil and water
Sustainable Practices and Alternatives
Current sustainability initiatives focus on:
- Developing more efficient application methods
- Exploring alternative compounds with lower environmental impact
- Implementing better waste management protocols
Market Analysis and Future Trends of FeSO4
The global market for FeSO4 has shown steady growth, driven by increasing demand across various sectors.
Market Statistics and Growth Projections
Key market insights include:
- Annual growth rate: 4.5% (2020-2025)
- Major producers: China, India, United States
- Primary application sectors: agriculture, water treatment, pharmaceuticals
Emerging Applications and Innovations
Recent developments focus on:
- Advanced water treatment technologies
- New formulations for medical applications
- Environmentally-friendly production methods
Conclusion and Final Thoughts
Throughout this comprehensive guide, we have explored the multifaceted nature of FeSO4 compound name and its significance in various fields. From its fundamental chemical properties to its diverse applications in industry, medicine, and agriculture, FeSO4 proves to be an indispensable compound in modern science and technology.
The importance of understanding FeSO4 extends beyond its chemical name, encompassing its role in environmental management, health care, and sustainable practices. As we continue to develop new applications and improve existing ones, the responsible use of FeSO4 becomes increasingly crucial for maintaining ecological balance while meeting industrial and medical needs.
We encourage readers to share their thoughts and experiences with FeSO4 in the comments section below. Whether you're a professional working with this compound or simply interested in chemistry, your insights could provide valuable perspectives for others. For more detailed information on related topics, consider exploring our other articles on chemical compounds and their applications in various industries.